- Home
- Industry
- Products
- Services
- Cloud Platform
Enquire Now

Enquire Now

To provide prompt, consistent, reliable and cost-effective quality services to
various sectors worldwide, maintaining confidentiality and integrity
asis Infotech is a leading application software company, with many industry specific products underits flagship.
Oasis lnfotech has been instrumental in developing a wide range of software products to computerize the whole range of manufacturing, analytical and other activities.
Oasis Infotech has been pioneer in Laboratory Information Management System and was the first to introduce LIMS concept in India in the year 1988.
It has successfully undertaken various assignments of software development, implementation and consultation for various industries including many multinationals in India and abroad.
Business ready software solutions with Regulatory Compliance
Cost effective solutions vis-a-vis any other company providingsuch solutions
Proven capabilities and commitment for on-going services,future support
Over 100 implementations in LIMS space, largest clientelein this segment
Highly reliable, consistent services
100% success rate of implementation
Implementations keeping in view the regulations as well as the practices
Strong team of QA, QC experts and IT experts: backing for last over 20 years
Backed with 35 years pharma industry experience and knowledge
Pharmaceutical industry
Chemicals industry
Bulk drugs industry
Consumer health care units
Cosmetics industry
Contract research organizations
R&D laboratories
Commercial testing laboratories
QA, QC laboratories
Textile industry
Petrochemicals industry
Food testing, environmental laboratories
MNCs, SMEs
Large scale companies
Govt. of India Drugs Testing Laboratory
Public Sector Undertakings (PSUs)
The right software solution for automation of analytical, R&D, QA, QC, manufacturing and other activities
Industry specific software solutions
Oasis has formed technology partnerships with premier hardware platform, software, and service organizations to optimise the value of its LIMS solutions and improve its customer’s productivity and efficiency.
SAP AG, Integration Partner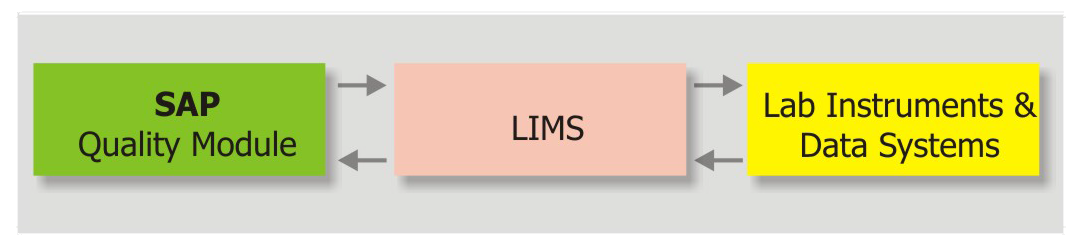
Integration setup : LIMS, SAP/ERP & Instruments
Oasis, a leading provider of laboratory information management systems (LIMS), and has over 21 years of experience in the LIMS market.
Oasis, a leading provider of laboratory information management systems (LIMS), and has over 21 years of experience in the LIMS market. We develop and market custom designed and configurable LIMS software solutions trade-named OasisLIMS.
OasisLIMS manages the collection, processing, storage, retrieval and analysis of information generated in laboratories. Our software improves the reliability of sampling processes, supports compliance with regulations and industry standards, provides comprehensive reporting, monitoring and analysis capabilities, and enables our customers to manage their globally distributed laboratories more effectively.
OasisLIMS software is used by hundreds of laboratories in over many countries around the world. Our strongest presence is in Indian reason. The adaptable nature of our software allows us to offer solutions to customers in a wide range of industries and in multiple disciplines, but primarily in quality control and assurance, testing and monitoring, and research and development.
The primary users of OasisLIMS are pharmaceutical manufacturing, API manufacturing, Chemical Plants and life sciences organizations.
OasisLIMS‘s vision is to integrate all the information a laboratory creates into a single, web-based platform, while providing the means to manage a wide range of laboratory processes and workflows.
Following this strategy, we launched an entirely web-based LIMS a decade back and are regularly adding strategic capabilities to that powerful platform.
We introduced OasisLIMS O-Link Server SDMS, an integrated instrument interface solution capable of parsing and managing unstructured data together with structured (LIMS) data. Leveraging these two technologies, we made another step towards a unified platform for the paperless laboratory by developing the OasisLIMS Electronic Notebook to support automated lab operation.
Pharmaceutical industry
Chemicals industry
Bulk drugs industry
Consumer health care units
Cosmetics industry
Contract research organizations
R&D laboratories
Commercial testing laboratories
QA, QC laboratories
Textile industry
Petrochemicals industry
Food testing, environmental laboratories
MNCs, SMEs
Large scale companies
Govt. of India Drugs Testing Laboratory
Public Sector Undertakings (PSUs)
The right software solution for automation of analytical, R&D, QA, QC, manufacturing and other activities
Industry specific software solutions
Business ready software solutions with Regulatory Compliance
Cost effective solutions vis-a-vis any other company providing such solutions
Proven capabilities and commitment for ongoing services, future support
Over 100 implementations in LIMS space, largest clientele in this segment
Highly reliable, consistent services
100% success rate of implementation
Implementations keeping in view the regulations as well as the practices
Strong team of QA, QC experts and IT experts: backing for last over 21 years
Backed with 35 years pharma industry experience and knowledge
We have been a premier company to provide LIMS for more than 21 years.
Our core focus is on the development and deployment of commercial laboratory informatics and we have devoted all of our research and development efforts to developing industry-leading solutions in this field.
With hundreds of implementations in multiple industries around the globe, we have developed proven solutions for a wide range of markets, including government, life science and manufacturing.
21-years of expertise in delivering solutions to the needs of our customers, our team has accumulated and continue to accumulate industry-specific know-how and best practices, earning OasisLIMS a reputation for high quality service. We have a long track record of enabling our customers to easily upgrade to new versions, which safeguards our customers‘ investments.
We are a leading innovator in the laboratory informatics industry. We were among the first companies in India to offer custom designed and configurable LIMS solutions and we have repeatedly been early-to-market with advanced solutions lineup.
We were among the first to offer a PC-based client/server LIMS solution, following, our web-based LIMS solution that addresses evolving market trends towards centralized LIMS solutions.
As companies have grown and become multi-plant set-ups, the need for centralized data management and analysis has become critical to a wide range of potential customers. Our web-based LIMS facilitates multi-plant deployment and centralized application management, with no need for desktop downloads or client machine configurations.
Our advanced solutions enable enhanced data management and decision-making within the laboratory throughout the organization. By leveraging API’s and extensible markup language, or XML, and other web-services, we are able to offer a solution with enhanced interoperability and user experience, presenting real-time information in an easy-to-use graphical format.
For laboratories concerned about regulatory compliance, operational efficiency and optimizing information resources – OasisLIMS offers a unique integrated platform of exceptional in-depth working. Our entirely web-based platform includes not only an award-winning laboratory information management system (LIMS), but also advanced solutions for Instruments data capturing and management, electronic laboratory notebooks (ELN), Research and Development (R&D), environmental monitoring (EM) and much more.
Deploying OasisLIMS means guaranteeing regulatory compliance and ensuring that decisions are based on accurate, up-to-the-minute information.
Our solutions are fully validated on GAMP guidelines and compliant with GxP, GLP, 21 CFR Part 11, MHRA, ISO 17025 etc.
OasisLIMS® has a 24 years track record and recognition of LIMS based solutions India-wide, since its inception, Oasis has devoted itself solely to developing world-class software product, the result of hundreds of man-years of focused research and development, a flexible LIMS platform that is easily configured to match the dynamic processes/standards of any lab. The company solutions support …
Oasis basic policy is to comply with regulations by integrating data management for all instruments used in the laboratory, including chromatographs, mass spectrometers (HPLC, GC, LC –MS, GC-MS), spectrophotometers (UV, FTIR etc.), total organic carbon analyzers (TOC), thermal analyzers, and balances.
Oasis products provide solutions for regulatory compliance of all lab analysis data from chromatographs, spectrophotometers to balances. O-Link supports networking for all analytical instruments to enhance workflow efficiency and data reliability.
Oasis regulatory compliance network systems ensure data reliability, support audit trail, security and integrity.
It is important that the specification requirements meet the demands on the system and operating environment, and also incorporate the technical elements to satisfy part 11.
The documentation of plans procedures, and reports and appropriate review, approval, and management
O-link user administration comprises of the setting of rights group and assignment of rights to users, it enables easy setting of user access rights as well as rights group matched to each user’s required task. Functions such as these achieve effective user administration matched to laboratory operations from managerial tasks to data acquisition operations.
Features functions for setting an audit trail to ensure data reliability and security features include various settings, such as setting the length, expiration date and complexity of passwords for user accounts, setting the lockout function to prevent illegal access, and registering settings for the deletion and alteration of registered users, to enable highly secure system operation.
The changes history of method files is managed by the audit trail, application of audit trail to all methods can also be set in security policy settings. This prevents inconsistencies in compliance with regulations.